Call us: (+856-21) 314930 |
About PDC3
PDC3 is a state-owned pharmaceutical enterprise specializing in pharmaceutical research, development, and production. We focus on medicines for treating anemia, vitamins, antipyretics, analgesics, and cough medications.
Since our establishment in 1987, we have been committed to producing high-quality pharmaceutical products that meet international standards. Our dedication to research and development has positioned us as a leader in the pharmaceutical industry.
We continuously strive to improve our manufacturing processes and implement stringent quality control measures. Our facility operates in accordance with ISO standards and Good Manufacturing Practice (GMP) guidelines, ensuring the safety and efficacy of our products.
Mission & Vision
Our Mission
Medical Innovation Inspired By Lao Ideas and creatively presented to the world.
🎯
Our Vision
Corporate motto are the principles that guide behavior within our company
🔭
Corporate Motto: Principles That Guide Our Behavior
01
Professionalism
02
Total Quality
03
Innovation
04
Unity
Leadership Team

Dr. Lahounh CHANTHABOUT
Managing Director

Dr. Phoukhong Chommala
Deputy Managing Director

Mr. Khamphouy INTHAVONG
Vice Managing Director

Dr. Manivone SISAVENGSOUK
Vice Managing Director
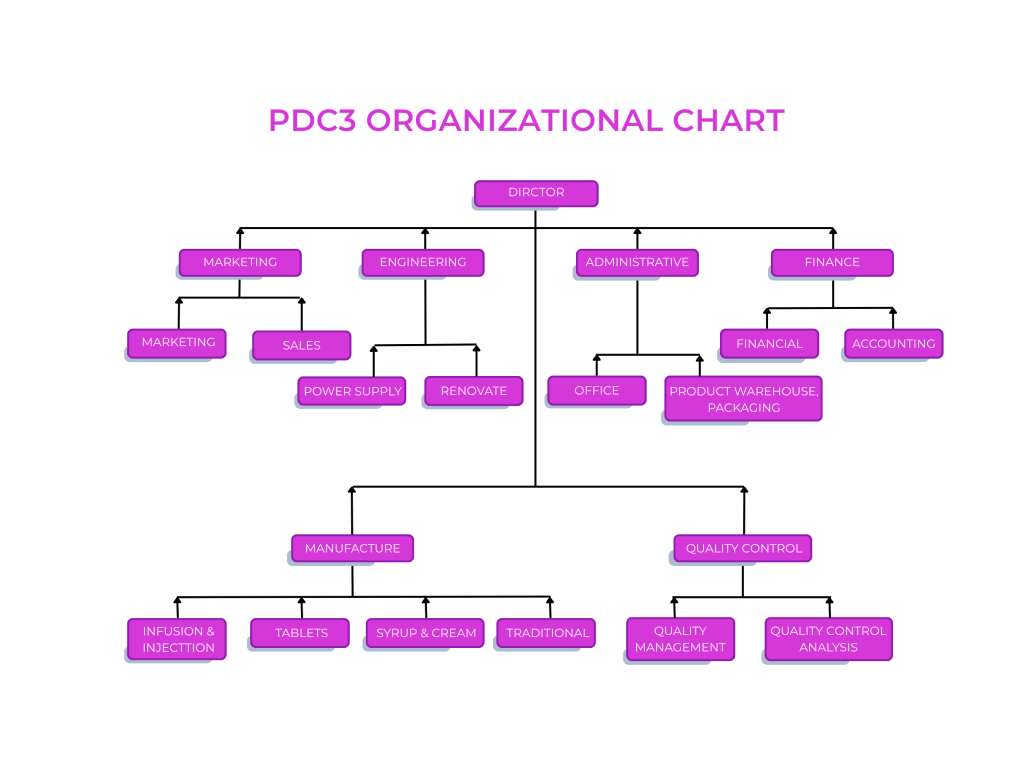
PDC3 ORGANIZATIONAL CHART
Certifications & Quality Standards
We continuously implement stringent quality control measures and maintain international certifications to ensure the safety and efficacy of our products.
Our Journey
1987
1983
1987-1990
2000
2006
2011-2015
2018
Company Statistics
Production Capacity
Certifications & Quality Standards
1983
Technical cooperation agreement with JICA and Macao Iakala for pharmaceutical production
1987
Establishment of pharmaceutical research and production facility
1988
Installation of modern production equipment and quality control systems
2000
Major facility expansion with 100 million kip investment in modern equipment
2001
Implementation of advanced pharmaceutical production technologies
2006
Achieved ISO certification and QA/QC standards including Kaldect Tea, Dechlosal Tea, and Aromatic products
2011-2015
Obtained GMP certification for pharmaceutical production facilities
2018
Technology partnership with TSUJIKO Co Ltd for quality enhancement and product development
Strategic Partnerships
2018
Partnership agreement with Tsujiko Co., Ltd (Japan) and Consulus Pte Co. Ltd (Singapore) for technical cooperation and business development
2010-2015
Collaboration with KBN (Sino-American Kunming Baker Norton Pharmaceutical Co.) for production and distribution
1995-2004
Partnership with Labiofam SA for production of medicines, including pharmaceutical research and development of traditional and modern medicines









